Identification of Polar Volatile Organic Compounds in Consumer Products and Common Micro-Environments
INTRODUCTION
Previous studies have shown that common personal activities(1) and emissions from building materials(2) or consumer products, particularly in enclosed spaces (micro-environments)(3) can elevate exposures to a number of toxic and carcinogenic volatile organic compounds (VOCs). These earlier studies investigated about 25 personal activities, 50 building materials, and 32 micro-environments for a set of about 30 VOCs, mostly nonpolar aliphatic, aromatic, and halogenated compounds. These are of course only a small number of the hundreds or thousands of activities, materials, and micro-environments people encounter daily, and the chemicals are representative of only a few of the many classes of chemicals encountered daily.
In an attempt to broaden the number of micro-environments and chemical classes studied, the U.S. EPA sponsored a study of polar chemicals emitted from 31 consumer products and 16 micro-environments. Polar chemicals such as alcohols, aldehydes, esters, and ketones are of interest because of their odorous and irritant properties. Complaints of odors and eye, nose, and throat irritation are often encountered, particularly in the office environment, as part of a complex of symptoms known as Sick Building Syndrome (SBS)(4). Some people may be unusually sensitive to odors or irritant properties of these chemicals (chemical sensitivity)(5). Both SBS and chemical sensitivity may be widespread enough to have significant effects on the country's productivity and health care costs.
STUDY DESIGN
The study had three objectives:
- Determine whether canister collection followed by GC-MS analysis would be capable of identifying (and, if possible, quantifying) polar VOCs collected in micro-environments and in the headspace of products.
- Identify (and, if possible, quantify) all polar VOCs emitted by selected fragrance products.
- Investigate micro-environments containing these products for the presence of these polar (as well as nonpolar) VOCs.
The approach was to obtain standards for the various chemicals likely to be used in fragrance products and to determine whether the chemicals could be recovered quantitatively from the evacuated canister. If so, the products and micro-environments would be investigated quantitatively using the canister to collect headspace and microenvironmental samples.
If canister recoveries were low or variable, the canister would be supplemented by a direct injection method for the headspace analyses. This would allow comparison of the chromatograms from the two methods and identification of chemicals that were not recovered sufficiently by the canister. GC-MS analysis would provide for identification of the chemicals. However, the amounts of the chemicals would be determined only in a semiquantitative fashion.
A complete description of the study is provided in an EPA report(6).
METHODS
Standards for 34 chemicals used in perfumes, soaps, and other scented products were obtained and loaded into evacuated Summa™ canisters at levels of about 50 ng/mL. The same standards were also used to form dilute solutions in methanol (about 50 ng/uL). The standards were analyzed both from the canister and through injection of the methanol solution into the glass injection port of the GC-MS system, which is described fully elsewhere(3). Recovery efficiencies were calculated by comparing the amount recovered from the canister to the amount recovered from the direct liquid injection.
The 31 scented product brands to be tested were chosen from a broad variety of product categories such as perfumes, soaps, and deodorants. Brand names were selected based on recommendations from persons who had experienced health symptoms or discomfort that they attributed to the product. Generally only one or two different brands were tested within a category. Since only one semiquantitative analysis was made for each sample, the results can not be interpreted as indicative of that sample's "actual" or "typical" composition; therefore brand names will not be revealed.
Scented products were tested using a headspace generation system. A small amount of the product was placed in a headspace purge vessel and then a stream of slightly humidified N2 gas was directed through a port in the vessel. The gas stream was collected by a 1.8L canister with a restrictive orifice or went directly to the analytical instrument. The transfer line from the headspace purge vessel to the cryotrap was heated to 50° C. For canister analyses the transfer line was not heated, to better evaluate the typical mode of analysis of canister air samples. No dryer to remove excess humidity was used in either case, in order not to lose the polar chemicals along with the water vapor.
Canisters were used to collect 1.8L grab samples from 15 commercial establishments expected to contain scented products (potpourri stores, craft and hobby stores, etc.) and a few homes.
RESULTS
Of the 34 fragrance standards tested, nine were not recoverable from the methanol solution: linalool, linalyl acetate, hydroxycitronellol, triethylamine, benzyl salicylate, hexyl cinnamaldehyde, mush ambrette, eugenol, and furfuryl propionate. Recoveries of most of the remaining 25 chemicals from the canister were generally poor, typically getting worse with higher boiling points and lower volatilities. Since fragrances are selected partly for their ability to remain associated with the person or product, they tend to have low volatilities. These results indicate that the higher boiling compounds used in fragrances may not be recovered very well from passivated canisters, and that the sampling methodology may need to be changed to one that can better capture and release these compounds.
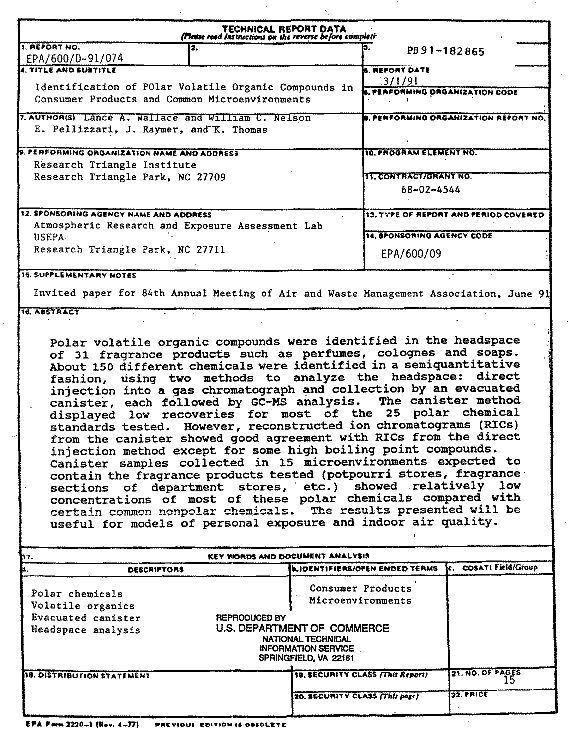
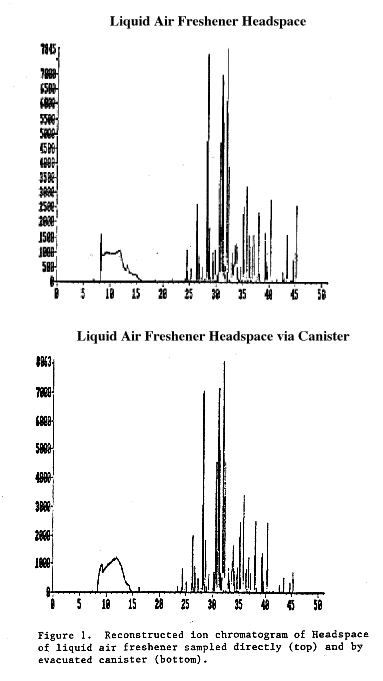
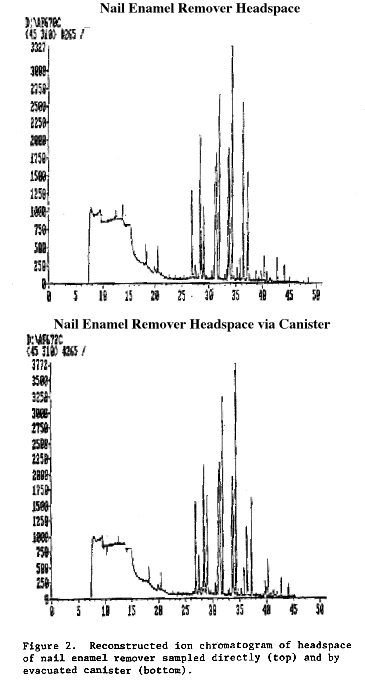
Because of the low recoveries for many compounds, the canister was supplemented, as planned, by a direct injection method for the headspace analyses. All 31 products were analyzed using the direct injection method. On 17 of these, the canister was also employed. It is interesting to note that canister recoveries appeared to improve when the much larger concentrations associated with the headspace of the scented products were sampled, as could be determined by comparing the headspace and canister chromatograms. Figures 1-3, typical of a number of these comparisons, show that for the products tested the canister matched the headspace chromatogram fairly well up to a retention time of 35-40 minutes, after which the response of the canister was degraded. Many of the fragrance standards (e.g., linalool) that had poor recoveries in the initial set of experiments showed up consistently in both the headspace and canister samples of the scented products.
All chemicals were identified but only semiquantitative estimates were provided for their concentrations in the microenvironmental and product samples. The chemicals with the highest concentrations (relative to the other chemicals) in each sample are listed in Table I.
A total of about 150 chemicals were identified in the 31 products, and about 100 chemicals in the 15 micro-environments. The chemicals appearing most often in the products and micro-environments are listed in Tables II and III.
DISCUSSION
The headspace analysis method employing direct injection through heated transfer lines with no dryer was capable of identifying some hundreds of polar and nonpolar VOCs emitted by the tested products. The method employing collection of headspace vapors in a canister, followed by nonheated transfer lines with no dryer, also gave useful semiquantitative results for many of these same polar and nonpolar chemicals, but with some degradation of performance noted for chemicals with higher boiling points. From previous studies, the point at which canister performance begins to degrade occurs roughly at the boiling point of n-dodecane(7). For chemicals with higher boiling points, a different collection medium such as Tenax may be more suitable, although this hypothesis needs to be tested in the case of the polar organics.
As can be seen from comparing Tables II and III, a different set of chemicals appeared to be found in the micro-environments compared with the scented products, despite the fact that many of these micro-environments were chosen because they contain these products. This may be due to the observed low recoveries that occur when the canister is employed in atmospheres with low concentrations of polar chemicals.
The 31 fragrance products tested contained a number of the same chemicals. Chemicals that appeared in more than half of these products included ethanol, limonene, linalool, B-phenethyl alcohol, and B-myrcene. Ethanol is an alcohol used as a solvent base for many of these preparations. Limonene is a terpene contained in citrus fruits, pine trees, etc. and is a very popular additive to perfumes, soaps, polishes, room air fresheners, soft drinks , and innumerable other products. Linalool is found in cinnamon and lavender. B-phenethyl alcohol is present in many flowers, and has a roselike scent. B-myrcene is found in bay leaves, verbena, and hops.
Many of the other chemicals found in the fragrance products are natural chemicals occurring in flowers, fruits, and trees(8). Their function in some cases appears to be to attract helpful insects such as bees; in other cases, the scents act as a.repellant (e.g., citronella). Few of these chemicals have been tasted for carcinogenicity, although some (e.g., a-pinene) are known mutagens and others (e.g., camphor) have known toxic effects at high concentrations(8). Limonene was tested for carcinogenicity and was observed to cause cancer in male rats, but not in mice or female rats(9).
Some of the brand names tested have been identified as being associated with mucous membrane irritation, weakness, or other symptoms in some people. If one or a few ingredients are responsible for the irritant health effects of some products, the results of the individual chemical analyses of these products (available on request from the authors) could be used to select likely individual chemicals for controlled experimental exposures.
The most common chemicals observed in the 15 micro-environments were toluene, methylene chloride, ethanol, 1,1 ,l-trichloroethane, silane compounds, a-pinene, isopropanol, xylenes, and undecane. Of these, only ethanol was among the five most common chemicals associated with the fragrance products. Toluene and xylenes are petroleum-based aromatic compounds widely used in paints, adhesives, and literally thousands of other products. Methylene chloride is a manmade halocarbon used in paint removers and many other solvents. l,l,l-trichloroethane is another halocarbon solvent used in hundreds of products including polishes and dry cleaning fluids. a-pinene is a terpene found in pine and many other woods, and is a popular additive (pine scent) to polishes, soaps, air fresheners, etc. Decane and undecane are straight-chain hydrocarbons found in paints, adhesives, and building materials.
CONCLUSIONS
The canister method tested showed low recoveries on most of the 34 fragrance standards tested at low concentrations. However, at the higher concentrations encountered in the headspace of fragrances, both the direct injection method and the canister method were capable of identifying scores of polar VOCs emitted from products. The most common of these polar VOCs were identified, and included alcohols, esters, and aldehydes. Since some of these polar VOCs are associated with irritation, odors, and other health and comfort concerns(4), identification without quantitative measurements may be useful in determining chemicals emitted by a suspect source. Some evidence of reduced canister recoveries for polar VOCs with high boiling points was noted; a different collection method may be required for polar VOCs with boiling points greater than that of n-dodecane.
Many of the chemicals detected in the fragrance products are naturally derived from plants(7). Although 150 different chemicals were detected in 31 such products, a rather small sat of these natural fragrances appeared in many of the products. Thus if one or more of these chemicals are responsible for the human health reactions to fragrant products reported by many(5), it could be possible to carry out a testing program on the set of 15-20 chemicals reported here as appearing repeatedly in these products. It would also be possible to use the methods described here to identify chemicals of interest in other products associated with health symptoms or comfort complaints.
DISCLAIMER
This paper is based on research sponsored by the Environmental Protection Agency but does not necessarily reflect EPA policy.
REFERENCES
- L.A. Wallace, E. Pellizzari, T. Hartwell, V. Davis, L. Michael, and R. Whitmore, "The Influence of Personal Activities on Exposure to Volatile Organic Compounds," Environ. Res. 50:37-55, 1989.
- L. Sheldon, L. Wallace, et al., Indoor Air Quality in Public Buildings: Vols. I and II USEPA, Research Triangle Park, NC EPA 600/S6-88/009a,b. 1988.
- E.D. Pellizzari, K.W. Thomas, J.H. Raymer, D.J. Smith, and S.D. Cooper, Breath measurements of Individuals Exposed to Chemicals During Personal Activities, Final Report, EPA Contract #68-02-4544, Work Assignment II-40, 1990.
- Molhave, L., Bach, B. and Pedersen, O. "Human Reactions to Low Concentrations of Volatile Organic Compounds," Environ Inter 12:167-175, 1986.
- Ashford, N.A. and Miller, C. Chemical Exposures: Low Levels and High Stakes. Van Nostrand Reinhold, New York. 1991.
- J.H. Raymer, E.D. Pellizzari, S.D. Cooper, N.P. Castillo, and K.W. Thomas, Evaluation of a Pharmacokinetic Model for Volatile Organic Compounds in Breath and of the Application of the Analytical Method to Polar VOCs, final report, EPA Contract #68-02-4544, Work Assignment #II-80, 1990.
- E.D. Pellizzari, K.W. Thomas, J.H. Raymer, D.J. Smith, and S.D. Cooper, Breath Measurements of Individuals Exposed to Chemicals During Personal Activities, Final Report on EPA contract 68-02-4544. Work Assignment II-40. 1990.
- The Merck Index, 11th Edition. Merck & Co., Rahway, NJ. 1990.
- National Toxicology Program, NTP Technical Report on the Toxicology and Carcinogenesis Studies of d-Limonene, National toxicology Program, Research, Triangle Park, NC. 1988.
Table I. Number of chemicals identified and principal chemicals present in products tested and locations sampled.
| Product | N(a) | Principal chemicals (b) |
| perfume #1 | 55 | linalool, limonene, ethanol, B-citronellol |
| perfume #2 | 24 | linalool, a-terpineol, limonene, benzyl acetate |
| perfume #3 | 28 | linalool, unknown, 1,8-cineole, neryl acetate |
| cologne #1 | 49 | limonene, linalool, B-phenethyl alcohol |
| cologne #2 | 45 | limonene, a-guaiene, C15H24 |
| bar soap #1 | 54 | limonene, a,B-pinene, linalool |
| bar soap #2 | 40 | limonene, linalool, other alcohols |
| shampoo | 25 | linalool, benzyl acetate, B-citronellol |
| hairspray #1 | 19 | fluor compounds, benzaldehyde, silane compound |
| hairspray #2 | 17 | unknown, butene, butane, ethanol, C11H24O, isopentone, a-terpineol, isobutane |
| shaving cream | 17 | limonene, propanal |
| after shave lotion | 19 | menthol, B-citronellol |
| solid deodorant #1 | 34 | hexamethylcyclotrisiloxane , trimethylsilane, limonene, linalool |
| solid deodorant #2 | 12 | limonene |
| spray deodorant | 26 | silane compounds, limonene |
| hand lotion | 22 | unknown, linalool |
| nail color | 18 | camphor, unknown, alcohol ? (c) |
| nail enamel remover #1 | 12 | benzyl alcohols, linalool, B-citronellol, limonene, B-phenethyl alcohol |
| nail enamel remover #2 | 14 | limonene, ethyl acetate, a-terpinolene, nerol, C10H18O |
| detergent powder | 20 | a-terpineol, linalool |
| bleach powder | 25 | a-terpineol, linalool, C12H2202 (acetate) |
| fabric softener #1 | 23 | benzyl acetate, limonene, y-methyl ionone, linalool, ester (?), ethanol |
| fabric softener #2 | 28 | ester (?), C12H2202 (acetate), linalool, a-terpineol, B-citronellol, C14H22O (alcohol) |
| dishwashing liquid #1 | 19 | limonene, ethanol, acetone |
| dishwashing liquid #2 | 15 | limonene, styrene |
| dishwasher detergent | 19 | terpinyl acetate |
| liquid air freshener | 29 | linalool, limonene, alcohol, Cl0H18O, Cl0H20O |
| solid air freshener | 24 | alcohols, limonene, Cl0H18O (?), camphor |
| spray air freshener | 16 | fluor compound, limonene, C15H24, ethanol |
| correction fluid | 16 | trichloroethylene, ethylene dichloride |
| paint remover | 8 | toluene, methylene chloride |
| department store | 25 | toluene, p-dichlorobenzene, ethanol |
| clothing store | 28 | ethanol, isopropanol |
| shopping mall | 29 | ethanol, isopropanol |
| potpourri shop | 33 | ethanol, toluene |
| craft/hobby store #1 | 31 | isopropanol, l,l,l-trichloroethane |
| craft/hobby store #2 | 31 | isopropanol, unknown |
| auto part shop | 30 | toluene, methylene chloride, tetrachloroethylene |
| tire shop | 17 | l,l,l-trichloroethane |
| tire warehouse | 27 | l,l,l-trichloroethanel |
| carpet store | 24 | 1,1,1-trichloroethane |
| grocery, detergents | 28 | limonene, tetrachloroethylene |
| grocery, pet foods | 18 | limonene, tetrachloroethylene |
| health club | 17 | ethanol, silane compound |
| room with air freshener | 14 | p-dichlorobenzene, ethanol |
| closet with cedar chips | 22 | ethanol, limonene |
| new shower curtain | 16 | decane, ethylene dichloride |
- (a) Number of chemicals identified in headspace or canister sample
- (b) In order of relative amounts
- (c) Identification tentative
Table II. 20 most common chemicals found in 31 fragrance products.
| CHEMICAL | N(a) |
| ethanol | 23 |
| limonene | 23 |
| linalool | 22 |
| B-phenethyl alcohol | 21 |
| B-myrcene | 17 |
| benzyl acetate | 15 |
| benzyl alcohol | 15 |
| benzaldehyde | 14 |
| a-terpineol | 14 |
| ocimene | 13 |
| B-citronellol | 13 |
| a-pinene | 12 |
| acetone | 11 |
| ethyl acetate | 11 |
| y-terpinene | 11 |
| 1,8-cineole | 10 |
| a-terpinolene | 9 |
| nerol | 9 |
| camphor | 8 |
| methylene chloride | 8 |
- (a) Number of times chemical identified in headspace of 31 products
Table III. 15 most common chemicals found in 15 micro-environments.
| CHEMICAL | N(a) |
| toluene | 15 |
| methylene chloride | 14 |
| ethanol | 12 |
| l,l,l-trichloroethane | 12 |
| silane compound | 12 |
| a-pinene | 11 |
| isopropanol | 11 |
| m,p-xylene | 11 |
| n-undecane | 11 |
| n-decane | 10 |
| limonene | 10 |
| chlorodifluoromethane | 9 |
| acetone | 9 |
| trimethylbenzene isomer | 8 |
| n-nonane | 7 |
- (a) Number of times chemical identified in 15 micro-environments
IMAGES
Lance A. Wallace
U.S. EPA
Atmospheric Research and Exposure Assessment Laboratory
Bldg 166, Bicher Road
Vint Hill Farms Station
Warrenton, VA 22186-5129
William C. Nelson
U.S. EPA
Atmospheric Research and Exposure Assessment Laboratory
Research Triangle Park, NC 27711
Edo Pellizzari
James H. Raymer
Kent W. Thomas
Research Triangle Institute
Research Triangle Park, NC 27709
Paper #A312 to be submitted for presentation at the 1991 Annual Meeting of the AWMA
March 1, 1991
(used by permission)








